
Corrosion is the chemical attack on a material leading eventually to its destruction if not stopped. It is caused by electrolytes, gases, solutions, or smelt. Corrosion occurs in different forms depending on the material under corrosive attack and the attacking agent. On metals, iron, for instance, its most visible manifestation is aerial or localized rust, such as needle holes in the surface. Crystalline corrosion of metals follows grain boundaries on surfaces. Corrosion is highly accelerated if the corroding material is in electrolytical contact with a more noble material. If this electrolytical contact is a liquid or humid substance, then corrosion is further accelerated. The reason is that the corroding material acts as anode (local cell) of a galvanic cell. Mechanical strain can accelerate corrosion as well.
Corrosion protection is accomplished by coating the vulnerable material with corrosion resistant dense films. Such coating can be other metals such as zinc or chrome, as well as glazing, for example enamel. Protective paint is a wide-spread measure and is accomplished by adding pigments such as red (minium) or white lead, or organic substances. Tight plastic wrap is used as well. Iron is protected through transformation into stainless steel by adding chrome, nickel, etc.
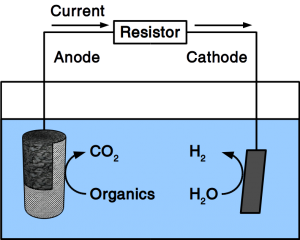
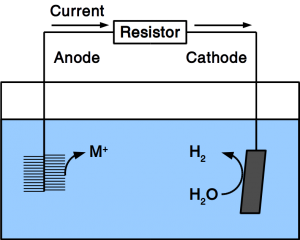
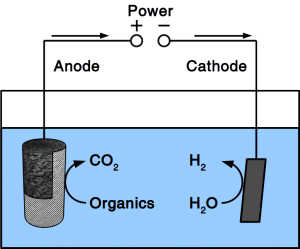
If the material is exposed to water permanently, cathodic protection is frequently employed. To accomplish cathodic protection, the vulnerable material is connected to sacrificial anodes such as rods or plates that dissolve over time. Alternatively, directed current is used in many applications. Our patent pending solution provides a microbial anode that uses organic matter in soil or sewer as sacrificial anode. Instead of dissolving the metal, organic matter is degraded by microbes.
Besides metals, natural (wood, silk) and artificial polymers (plastics, rubber) can corrode as well. Softwood is generally more resistant than hardwood. Weak acids usually do no harm to wood. However, corrosion protection of wood is accomplished by painting or soaking it using protective agents. Artificial polymers rarely corrode as quickly as metals and if they do, a protective agent is mixed into the polymer formula at the time of its synthesis.
This post is also available in Deutsch.




