Nitrogen compounds are essential for life on Earth. The use of fossil fuels and artificial fertilizers have led to a significant increase in reactive nitrogen available to the biosphere. This increase has far-reaching and well-researched effects on ecosystems, biodiversity and health. Air pollution can lead to premature deaths and nitrogen compounds may play an important role. Previous studies have only inadequately researched the effects of reactive nitrogen on the global climate system since industrialization.
A new study by the Max Planck Institute for Biogeochemistry in Jena is now closing this knowledge gap. The researchers modeled the terrestrial biosphere and global atmospheric distribution of nitrogen. They then combined the results with findings from atmospheric chemistry. This combination enabled them to come up with a new and comprehensive assessment of the climate impact of anthropogenic reactive nitrogen. The results were recently published in the renown scientific journal Nature.
Man emits a number of nitrogen compounds. Some of these, such as nitrous oxide (N2O, laughing gas), are greenhouse gases. Others, such as fine dust particles that reflect solar radiation, have a cooling effect on the climate. These effects were also described in the present the study. Significant warming due to increasing concentrations of the greenhouse gases nitrous oxide and ozone (O3) were reported. In contrast, several processes that contribute to the cooling effect of nitrogen were also described. In addition to particulate matter, these processes include chemical reactions that lead to a shorter residence time of the greenhouse gas methane in the atmosphere, as well as an increased uptake of carbon dioxide (CO2) by the terrestrial biosphere due to the fertilizing effect of nitrogen.
If all global warming and cooling processes caused by the reactive nitrogen are combined, a net cooling effect is the result. This new result suggests that nitrogen emissions have compensated for about one sixth of the global warming to date caused by the increase in CO2 over the industrial period.
The new results are also important for future strategies for nitrogen regulation in the context of climate protection policy. In most scenarios, nitrous oxide emissions from farming remained high due to the continued use of fertilizers in agriculture and thus the warming influence of this gas. Scenarios that are compatible with the climate goals of the Paris Agreement require an end to CO2 emissions from fossil fuels. This also reduces the release of reactive nitrogen from fossil sources and its harmful effects on health and biodiversity, but also eliminates its cooling effect. The researchers therefore expect a slightly warming contribution from total nitrogen for these climate protection scenarios, but this is far less than the warming from the unchecked consumption of fossil fuels.
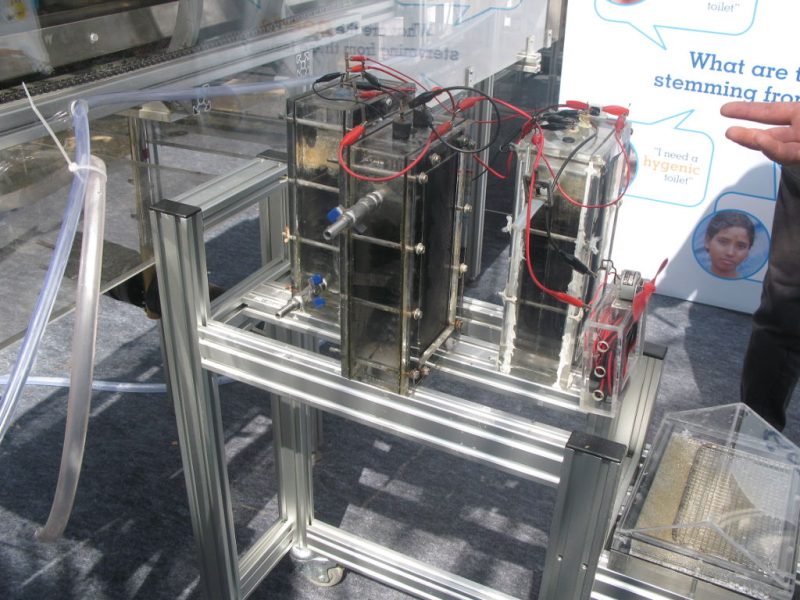
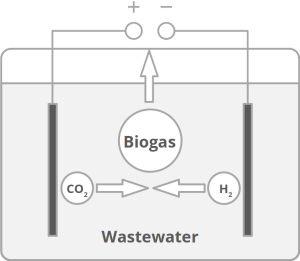
The study underlines the urgency of finally stopping emissions from fossil fuels and using fertilizers more specifically. This would not only slow down global warming, but also reduce the burden of harmful ozone and particulate matter concentrations for all of us in rural areas and in cities. New technologies are needed to reduce harmful nitrogen emissions while making good use of beneficial nitrogen. At Frontis Energy we have developed such a technology. Our patented process removes ammonia from wastewater while producing useful carbon neutral biogas. Using this technology, harmful nitrous oxide emissions can be reduced.
Picture: Smog over Guangzhou, China